Founded in 2014, Gongwin Biopharm Co., Ltd. (hereinafter referred to as Gongwin Biopharm) is a biotechnology pharmaceutical company based in Taiwan that focuses on the development of new anticancer drugs based on the concept of “integrity, innovation, professionalism and care”. Gongwin Biopharm breaks away from traditional cancer treatment thinking and combines minimally invasive treatment to develop new anticancer drugs with PTS as the main active ingredient, providing cancer patients with the option of directly eliminating tumors.

R & D
-Core Technology -
【PTS Targeted Tumor-Ablation】
【PTS Targeted Tumor-Ablation】
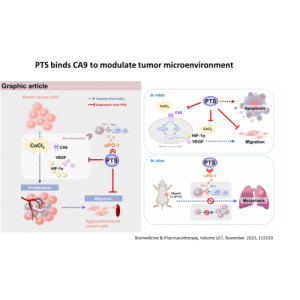
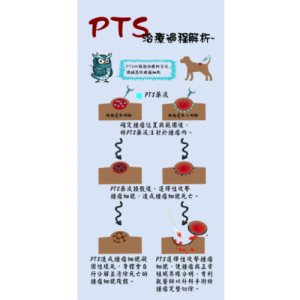
PTS is a chemical small molecule anticancer drug independently developed by Gongwin Biopharm. According to in vitro studies, PTS can penetrate the cancer cell membrane in a short period of time in contact with cancer cells and accumulate in the cytoplasm in a large amount, and the concentration of cancer cells is several tens of times relative to that in normal cells. When PTS enters cancer cells, it will rupture the intracellular lysosome, release lysozyme, and cause necrosis of cancer cells; PTS also promotes the release of cytochrome c from mitochondria in cancer cells ( Cytochrome c) inhibits ATP synthesis and induces cancer cells to develop apoptosis. This anti-cancer target and mechanism are very different from other anti-cancer drugs. Traditional chemotherapy drugs are mainly systemic cell poisoning. Patients often need to bear the side effects of drugs on normal cells, such as bone marrow suppression, hair loss, rash, vomiting, etc. The characteristic of PTS is that it acts directly on the tumor tissue as a direct guide. Because the scope of efficacy is specific and accurate, there is no such side effect that the patient can't bear.