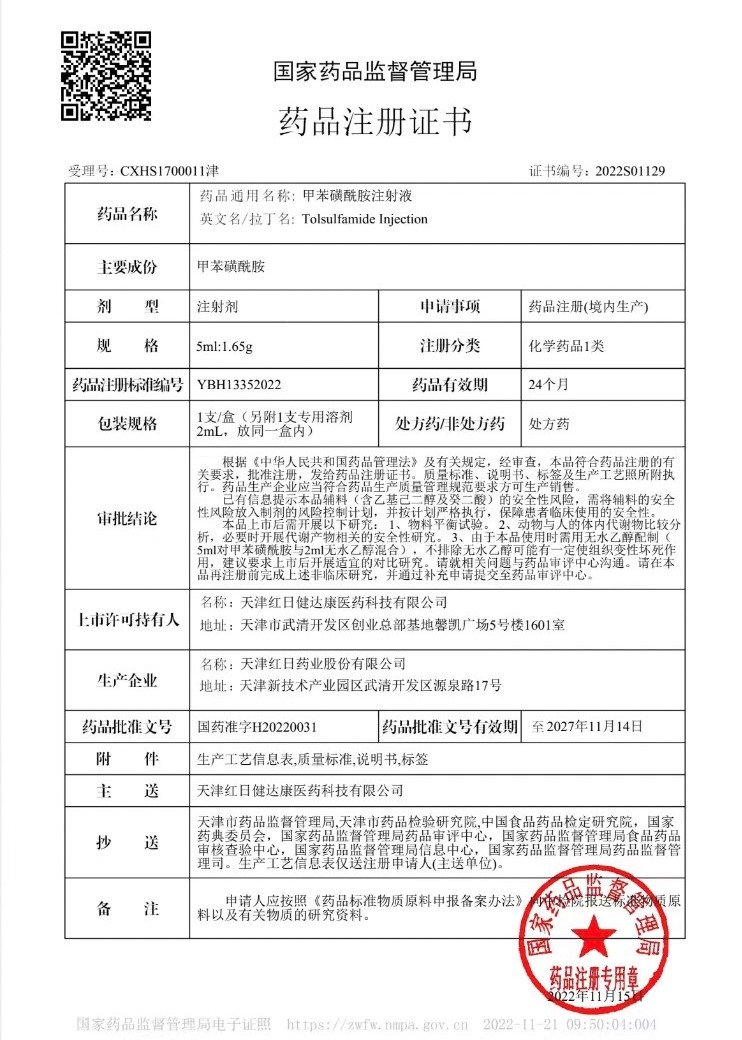
GongWin Biopharm is honored to announce that our PTS injection has been officially approved by CHINA NMPA (National Medical Products Administration) in November 2022.
After decades of research and years of registration progress, PTS ultimately received market approval in CHINA.
The small chemical molecule PTS was found in 1990s by our founder Dr. Shih and series of trials and research were conducted since then. Under NMPA’s supervision, PTS has been listed in CHINA pharmacopeia in 2020, and relevant dossiers were summited to CFDA for further review and inspection. In 2022, PTS earned the approval, which should initiate the post market clinical use shortly afterwards. GongWin has devoted ourselves in the treatment for tumor ablation since 1990s, and we sincerely believe that PTS would bring another hope for cancer patients. With the connections we have in China, PTS would bring a brand new treatment options for physicians and patients.