Introduction of PTS Trial Drugs and Core Technology of
"Minimally Invasive Targeted Tumor Ablation"
- PTS (para-toluenesulfonamide) is a chemical small-molecule tumor ablation drug independently developed by Gongwin Biopharm. Compared with systematic therapy, PTS has the advantage of a faster effect on the site of local drug application, and its advantages; when compared with physical therapy, PTS has advantages of stronger selectivity to tumor cells and less damage to normal cells. Its anti-cancer effect target and mechanism are completely different from other anti-cancer drugs.
- Three characteristics of "Minimally Invasive Targeted Tumor Ablation" core technology:
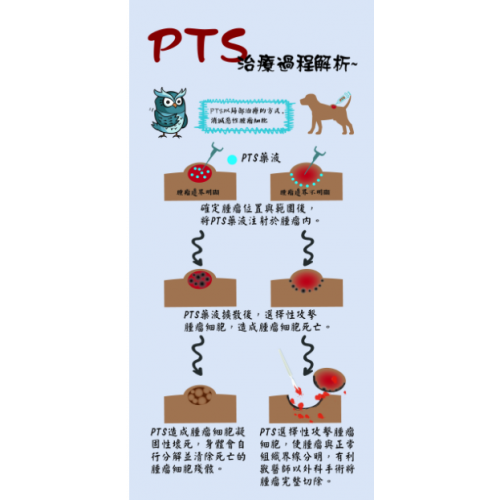
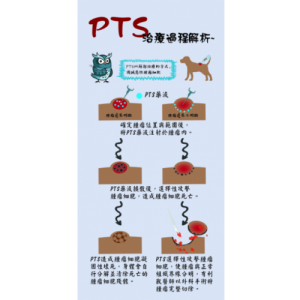
1. Selectivity: PTS causes tumor cell necrosis within 24 hours after injection through minimally invasive interventional, yet it causes minimal damage to adjacent normal cells.
2. Safety: According to a series of clinical trials, it is discovered that PTS does not cause side effects which are common to traditional chemotherapy drugs. In addition, it is relatively safe to inject PTS directly into cancer cells through image positioning technology.
3. Ablation: In a series of clinical trials on a variety of solid tumors, PTS was proven to have efficacy in killing malignant tumors, including head and neck cancer, breast cancer, lung cancer, liver cancer, etc.; meanwhile, it can reduce the accumulation of malignant tumors and produce margin edges between tumor tissues and normal tissues to facilitate subsequent surgical resection.
.png)