[Background]
We have completed a phase III study to treat severe MAO (≥1/2 trachea obstruction, or ≥2/3 obstruction on one side bronchus) by PTS intratumoral injection guided with bronchoscopy. Total 90 patients were enrolled.
[Treatment & Results]
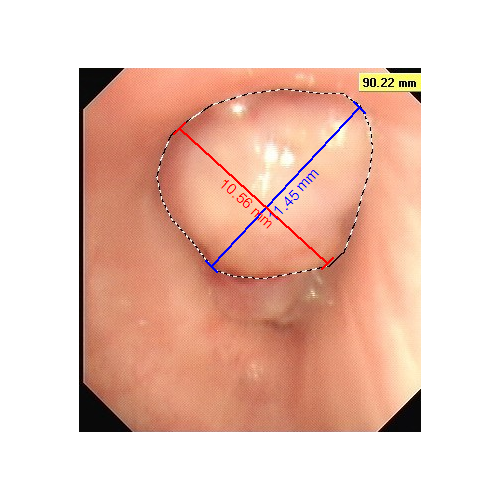
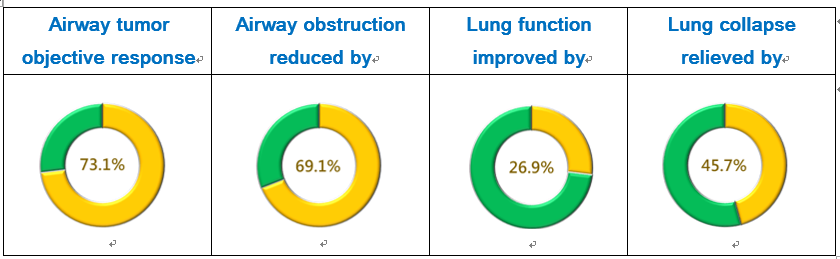
PTS intratumoral injection leads to tumor necrosis, so after the first injection, necrotic tissue was removed prior to the each of the following injections. At least 2 injections were conducted per week in each patient. After 2-6 weeks of the treatment, the airway objective response rate reached 73.1%. The airway obstruction rate was reduced by 69.1%. The lung function (the forced expiratory volume, FEV1) was improved by 26.9%, and 45.7% of the patients who had atelectasis (lung collapse) had relief (Fig. 1).
[Safety analysis]
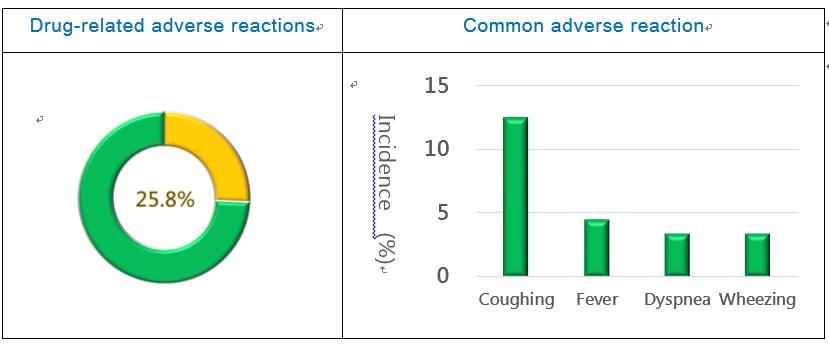
The incidence rate of adverse events in the phase III study was 64.0%. The incidence rate of drug-related adverse reactions was 25.8%, among which 82.6% was mild to moderate and 17.4% was severe (Fig. 2). The most common adverse reactions included: coughing (12.5%), fever (4.5%), dyspnea (3.4%), and wheezing (3.4%) (Fig. 3). A total of 7 serious adverse events were reported, among which 4 were drug-related, including: exacerbated airway stenosis, hemoptysis, and pulmonary heart failure.
Figure 2. PTS Phase III Study Safety Figure 3. PTS Phase III Study Safety (adverse reaction)
[Case report-Tumor size analysis]
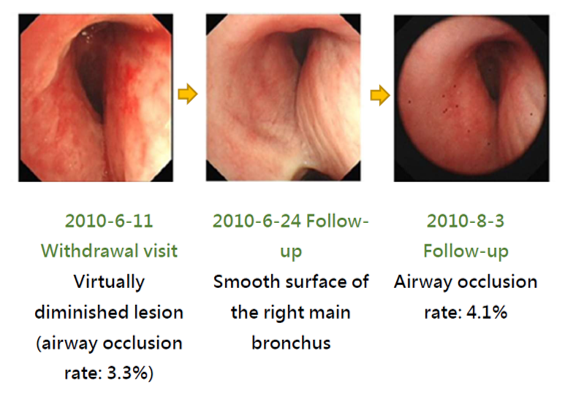
The treatment effect of PTS can be illustrated with the images of a case along the treatment course. This patient had a stage 4 squamous cell carcinoma at the right main bronchus. Prior to the treatment, the airway obstruction rate was 100%. After a total of 3 PTS injections (3-4 mL) and debridement in a 5-day course (6/4~6/9), the airway obstruction was significantly relieved and the obstruction rate was reduced to 3.3% (Fig. 4). PTS treatment has caused no damage to the neighboring tissues. The follow up in 2 months (8/3 follow-up) after the treatment showed a 4.1% airway obstruction rate (Fig. 4).
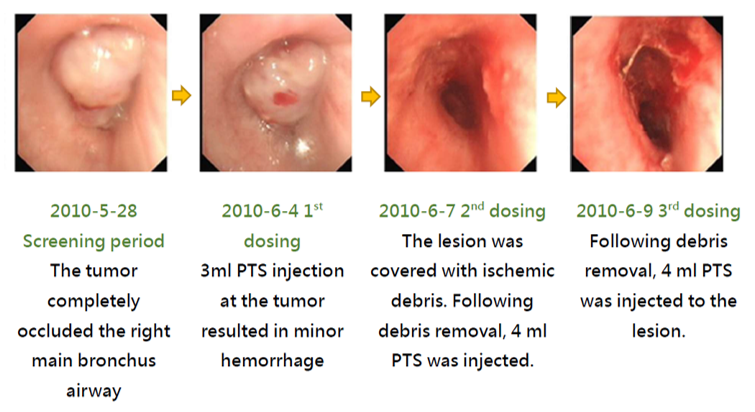
Figure 4. After PTS treatment –Airway occlusion rate analysis
Publication
Li et al. Lung Cancer (2016) 98: 43-50.