Treatment Result or Case
[Background]
In a subset analysis of our phase III study of PTS in China to treat NSCLC presenting with dyspnea due to severe MAO, 8 patients were diagnosed with inoperable tracheal ACC during enrollment. As part of the study, all 8 patients underwent intratumoral injection of PTS protocol.
[Treatment & Results]
PTS intratumoral injection leads to tumor necrosis, so after the first injection, necrotic tissue was removed prior to the each of the following injections. At least 2 injections were conducted per week in each patient and patients treated for 2-6 weeks. (Maximal cumulative dose of PTS was 10.0 ml for any single day.)
Two of the patients were lost to follow-up at the 30-day assessment. A tumor response rate of 100% (2 had complete and 4 had partial responses) was seen in the six patients followed at 30-days, and the airway obstruction was reduced by 68.7% of the 6 patients with ACC (Fig. 1). After PTS treatment, the dyspnea (BDI) improved by two times, and the lung function (FEV1, FVC) improved by two to three times (Fig. 2)
The study observation period was extended and treated patients were followed for 5 years post intervention. During the follow-up period, researchers were able to locate the 2 treated tracheal ACC patients lost at 30 days and included them in long-term outcome analysis. The 3-year survival rate was found to be 87.5% (7 of 8 patients), and the 5-year survival rate was 50% (4 of 8; median survival: 4.98 years) (Fig. 3).
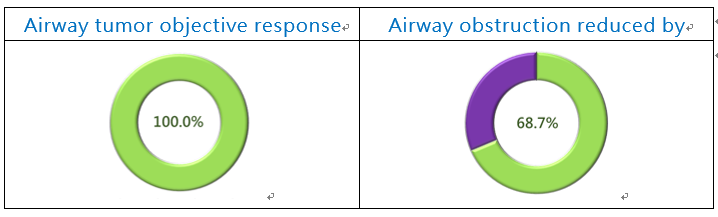
Figure 1. PTS Phase III - ACC Study Efficacy
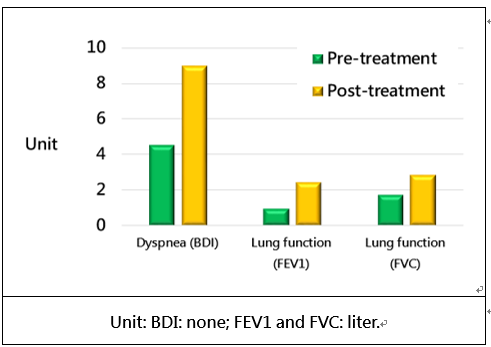
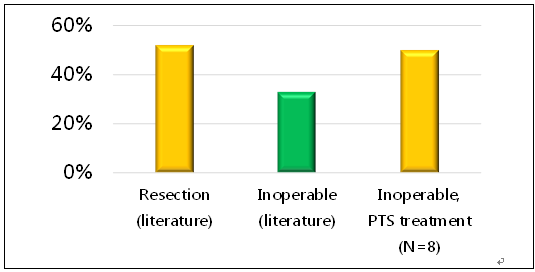
Figure 3. The 5-year survival rate of ACC patients.
[Safety analysis]
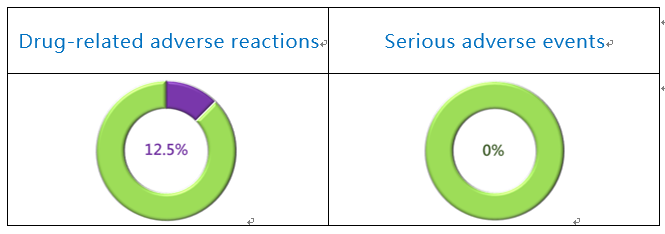
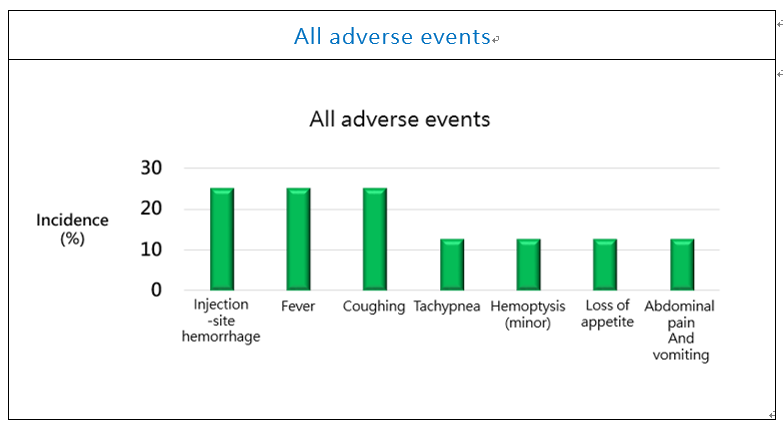
The incidence rate of adverse events in this subgroup was 75.0 %, and all were mild to moderate (100.0 %) (Fig. 4). The only 1 drug-related AE reported was mild tachypnea (12.5%). The adverse events included: injection-site hemorrhage (25%), fever (25%), coughing (25%), tachypnea (12.5%), hemoptysis (12.5%), loss of appetite (12.5%), and abdominal pain and vomiting (12.5%).
Figure 4. PTS Phase III Study Safety
Figure 5. PTS Phase III Study Safety (adverse events)
[Case report - tumor necrosis]
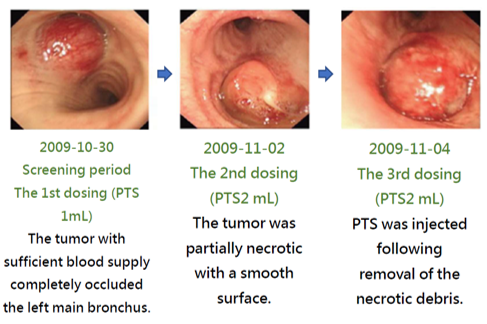
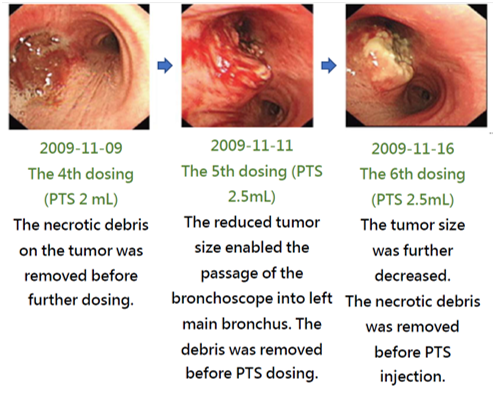
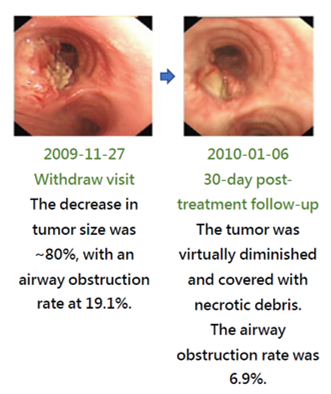
The treatment effect of PTS can be illustrated with the images of a case along the treatment course (Fig. 6). This patient had a stage 4 adenoid cystic carcinoma at the left main bronchus. Prior to the treatment, the airway obstruction rate was 100%. After a total of 6 PTS injections and debridement in a 19-day course, the airway obstruction was significantly relieved and the obstruction rate was reduced to 18.1%. PTS treatment has caused no damage to the neighboring tissues. The follow up in 2 months after the treatment showed a 6.9% airway obstruction rate. Therefore PTS may offer an effective choice for treating inoperable airway ACC which lacks effective medical treatment options.
Figure 6. Phase III in China - Ablation of tracheal tumors in ACC patients after PTS treatment
Publication
Guan WJ, et al. J Thorac. Dis. 2018;10(4): 2448-2455.