Treatment Results or Cases
[Background]
We have discovered the potential of MPE control by PTS intrapleural administration in 17 patients in a subgroup of the China phase II lung cancer study (94 NSCLC patients). The symptoms of MPE were relieved in most patients after intrapleural injection of PTS. We analyzed the efficacy of PTS for MPE patients, including the MPE control rate, treatment duration to take effect, duration of response, and safety.
[Treatment & Results]
As a subgroup study of the China phase II study, 17 non-small cell lung cancer (NSCLC) patients with MPE were enrolled and went through intercostal MPE drainage and intrapleural cavity administration of PTS. All NSCLC patients had underlying malignancy including adenocarcinoma (n=9), squamous cell carcinoma (n=2), and unspecified lung cancer (n=6). The patients received both drainage of pulmonary fluid and intrapleural injection of PTS. PTS injections were administered 1-3 times per week for 2-4 weeks according to the patient’s condition.
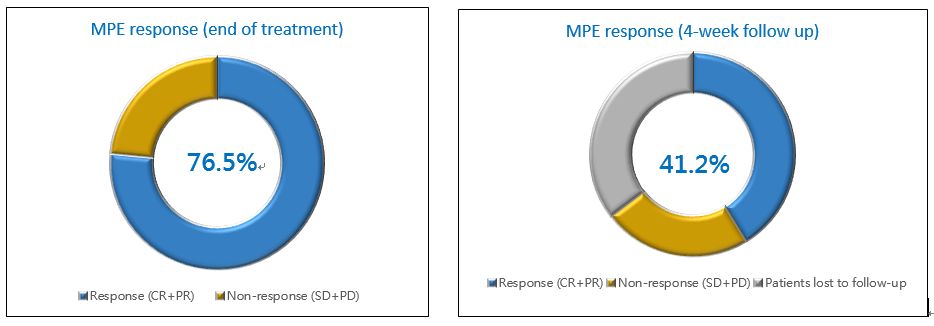
The primary endpoint for PTS to manage MPE was the MPE response which is shown below along with treatment and efficacy summary (Table 1). The data show that most patients were relieved (76.5%) at the end of PTS treatment, and 41.2% (7 of 17) of the patients had MPE response (6 CR, 1 PR) at 4 weeks after treatment. Also, PTS reduced the average MPE volume from baseline by a range of 24.1-67.2% for at least 30 days (manuscript in preparation).
Based on clinical data, we expect that these results support that PTS is worth further comprehensive clinical studies, and may be considered a patient-friendly option for MPE control in the near future.
Table 1. Remission of 17 MPE patients (decreased lung fluid)
[1]: complete response (CR): disappearance of MPE; partial response (PR): MPE volume reduces by over 50% compared to baseline; stable disease (SD): MPE volume changes from baseline by between -50% to +25%; progressive disease (PD): MPE volume increases by at least 25% compared to baseline.
[2]: 6 of the 17 MPE patients did not attend the visit at 4 weeks after PTS treatment, and their condition of MPE were unknown.
Figure 1. PTS Phase II Study Efficacy
[Safety analysis]
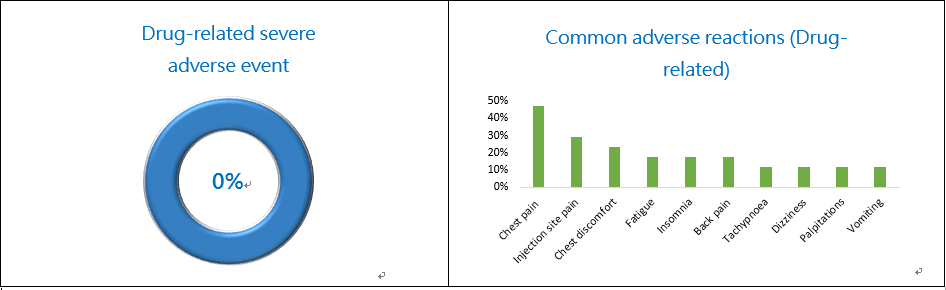
For safety, the detailed adverse event (AE) summary is shown below (Fig. 2). The most common AEs were mild to moderate chest pain (47.06%), injection site pain(29.41%), fatigue (17.65%), insomnia(17.65%)and back pain(17.65%)(Fig. 3), etc. No fever or infection was observed. No drug-related severe adverse event (SAE) was found. According to the clinical results, PTS is a safety drug for MPE treatment and MPE patients may have a better quality of life (less side effects) after PTS treatment.
Figure 2. PTS Phase III Study Safety Figure 3. PTS Phase II Study Safety (adverse reaction)

.PNG)