
Treatment Result or Case
Gongwin Biopharm Holdings Co., Ltd. has completed the liver cancer Phase II clinical trial in China. Data from patient treatment results show that PTS have significant effects in the treatment of advanced liver cancer. According to government statistics, liver cancer in Taiwan is the second leading cause of cancer deaths, and 11,225 people suffered from liver cancer in 2017. For HCC patients in Taiwan, Gongwin has initiated a phase II clinical trial in Taiwan, and we continue to recruit HCC participants.
[Background]
We have completed a phase II study in China to treat advanced liver cancer (primary or secondary liver cancer, unresectable or with residual lesion after transarterial embolization) with PTS via percutaneous intratumoral injection. A total of 83 patients were enrolled.
[Treatment & Results]
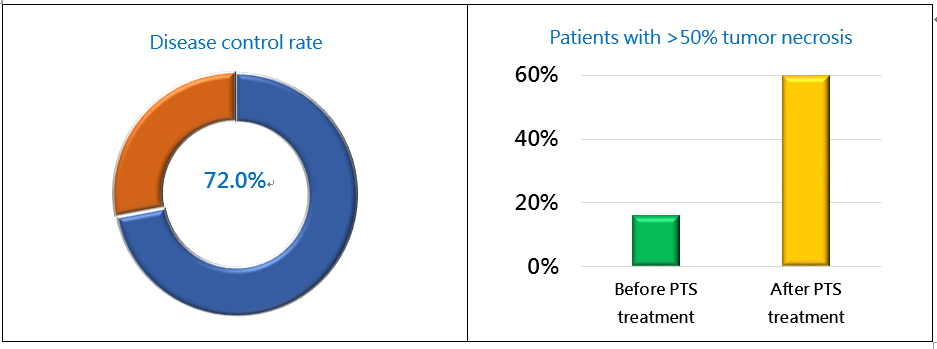
The enrolled patients received 1-3 PTS injections per week. The dose limit was 10 mL per treatment visit. The 83 patients received 1-10 injections according to their condition (an average of 4 injections). PTS intratumoral injection led to gradual ablation and necrosis of the tumor, which was spontaneously absorbed by body. Therefore the short-term efficacy assessment revealed stable in tumor size but increased tumor necrosis area of the treated lesion. At 4 weeks after the PTS treatment, the disease control rate (DCR by RECIST) was 72.0%, and the percent of patients with >50% tumor necrosis increased from 16.0% to 60.0% (Fig. 1). Based on clinical data, PTS has significant effects in liver cancer treatment.
Figure 1. PTS Phase II – Liver Cancer Study Efficacy
[Safety analysis]
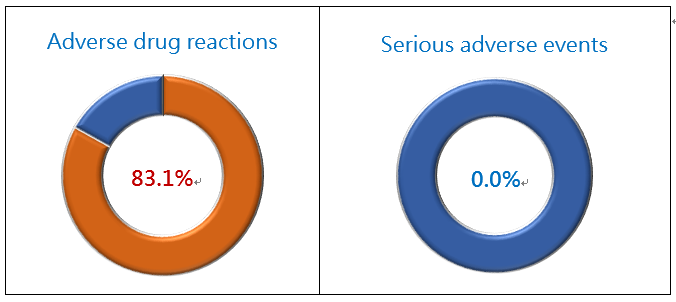
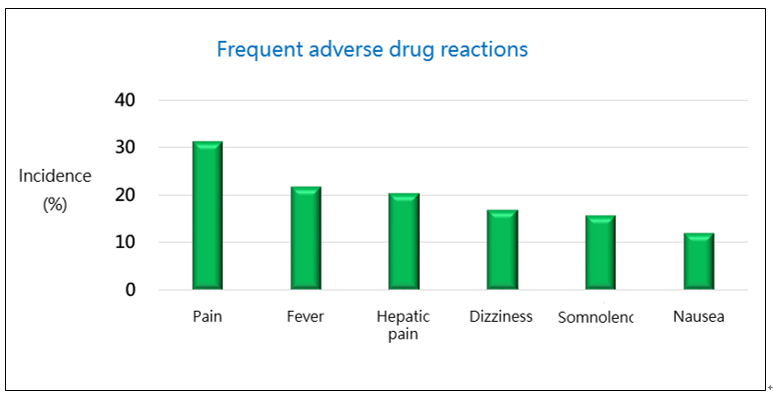
The incidence rate of adverse drug reactions (ADR) in this study was 83.1% (69/83), and the incidence of ADRs that required medical intervention for relief was 41.0% (34/83). There was no serious adverse event (SAE) reported (Fig. 2). The frequent (incidence >10%) ADRs included: pain (31.3%), fever (21.7%), hepatic pain (20.5%), dizziness (16.9%), somnolence (15.7%), and nausea (12.1%). No side effects like chemotherapies, such as bone marrow suppression, leukopenia, and hair loss, were reported in this study (Fig. 3). Therefore, PTS has milder side effects, and patients may have a better quality of life after PTS treatment.
Figure 2. PTS Phase II Liver Cancer Study Safety
Figure 3. PTS Phase II Liver Cancer Study – frequent adverse drug reactions.
[Case report]
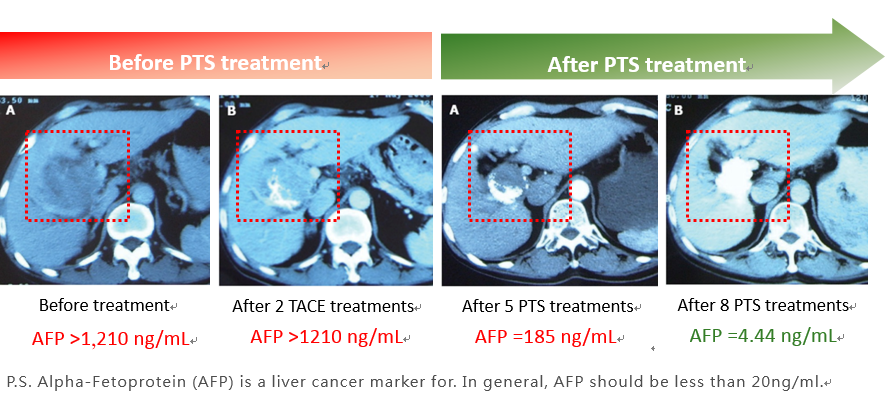
According to our clinical study conducted in China [1], one subject with portal vein invasion as late stage HCC shows limited efficacy after two TACE treatments, and no difference in AFP (Alpha-Fetoprotein, liver cancer marker) comparing to the level before TACE treatment. The subject is then receiving 5 PTS intratumoral injections, the result shows not only the tumor size decreased but also AFP. After receiving 8 PTS treatments in total, the tumor reveals necrotic effect, AFP returns to normal, and no recurrence for four years.
.png)
2. HCC Phase II in Taiwan
In 2018, we have been granted a Phase II, dose randomized, open-label clinical trial in Taiwan. The goal is to evaluate the safety and efficacy of PTS treating inoperable liver cancer patients or patients not suitable for currently available loco-regional ablation therapies. This Phase II trial is actively recruiting subjects in three medical centers located in northern Taiwan. Please refer to the HCC Phase II information for eligibility criteria of participants (Link here).
.png) Click the link and enter the keyword: type PTS100
Click the link and enter the keyword: type PTS100
.png)

.PNG)