Journal:Anti-Cancer Drugs 2015
Title:Para-toluenesulfonamide induces tongue squamous cell carcinoma cell death through disturbing lysosomal stability
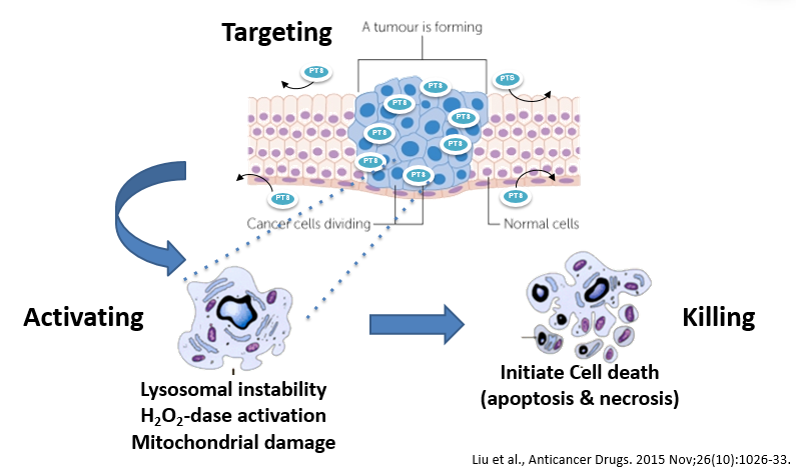
Abstract:
• Targeting:PTS can selectively penetrate the cancer cell membrane
To destroy the lysosomal membrane integrity, to increase the molecular permeability of the lysosomal membrane, to destroy the osmotic balance on both sides of the lysosomal membrane, to lead to lysosomal expansion and rupture, to leak the large amount of hydrolytic enzymes, to induce the damages of structure and function of cancer cells and finally cause cancer cells to rupture and die.
• Activating:Mitochondria dysfunction
PTS induces cytochrome c release from the mitochondria of cancer cells, activates caspase and related downstream molecules and eventually causes programmed cell death. The figure below shows that the concentration of cytochrome C increases with the concentration of PTS from 40umol/l to 80umol/l.
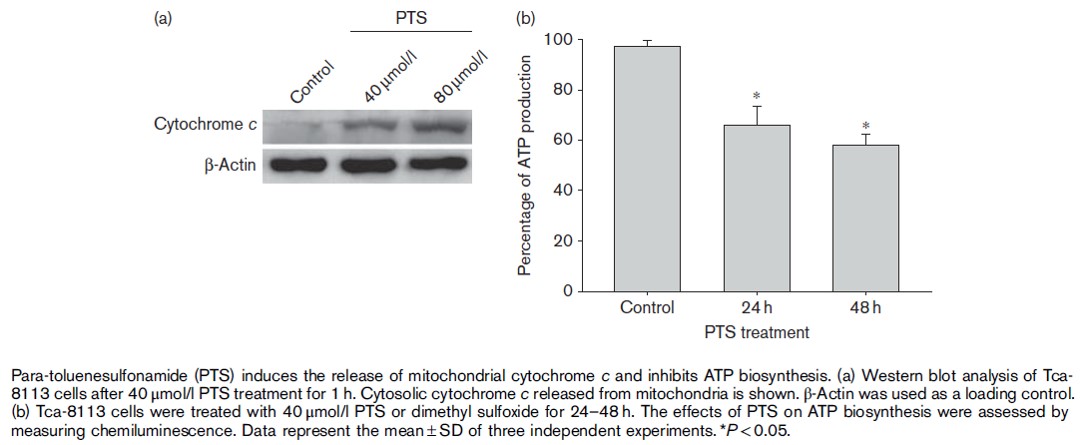
• Killing:Inhibition of mitochondrial ATP synthase activity in cancer cells
The PTS new drug can significantly inhibit the activity of mitochondrial ATP synthase in cancer cells, leading to dysfunction of mitochondrial energy synthesis, which further leads to cancer cell death.
Reference:Liu, Zhe; Liang, Chenyuan; Zhang, Zhuoyuan; More Anti-Cancer Drugs. 26(10):1026-1033, November 2015.
Journal:Frontiers in Pharmacology
Title:Para-toluenesulfonamide Induces Anti-Tumor Activity through Akt-Dependent and -Independent mTOR/p70S6K Pathway: Roles of Lipid Raft and Cholesterol Contents
.jpg)
Abstract:
Castration-resistant prostate cancer (CRPC) cells can resist many cellular stresses to ensure survival. There is an unmet medical need to fight against the multiple adaptive mechanisms in cells to achieve optimal treatment in patients. Para-toluenesulfonamide (PTS) is a small molecule that inhibited cell proliferation of PC-3 and DU-145, two CRPC cell lines, through p21- and p27-independent G1 arrest of cell cycle in which cyclin D1 was down-regulated and Rb phosphorylation was inhibited. PTS also induced a significant loss of mitochondrial membrane potential that was attributed to upregulation of both Bak and PUMA, two pro-apoptotic Bcl-2 family members, leading to apoptosis. PTS inhibited the phosphorylation of m-TOR, 4E-BP1, and p70S6K in both cell lines. Overexpression of constitutively active Akt rescued the inhibition of mTOR/p70S6K signaling in PC-3 cells indicating an Akt-dependent pathway. In contrast, Akt-independent effect was observed in DU-145 cells. Lipid rafts serve as functional platforms for multiple cellular signaling and trafficking processes. Both cell lines expressed raft-associated Akt, mTOR, and p70S6K. PTS induced decreases of expressions in both raft-associated total and phosphorylated forms of these kinases.PTS-induced inhibitory effects were rescued by supplement of cholesterol, an essential constituent in lipid raft, indicating a key role of cholesterol contents. Moreover, the tumor xenograft model showed that PTS inhibited tumor growth with a T/C (treatment/control) of 0.44 and a 56% inhibition of growth rate indicating the in vivo efficacy. In conclusion, the data suggest that PTS is an effective anti-tumor agent with in vitro and in vivo efficacies through inhibition of both Akt-dependent and -independent mTOR/p70S6K pathways. Moreover, disturbance of lipid raft and cholesterol contents may at least partly explain PTS-mediated anti-tumor mechanism.
Reference
Front. Pharmacol., 13 November 2018 | https://doi.org/10.3389/fphar.2018.01223

