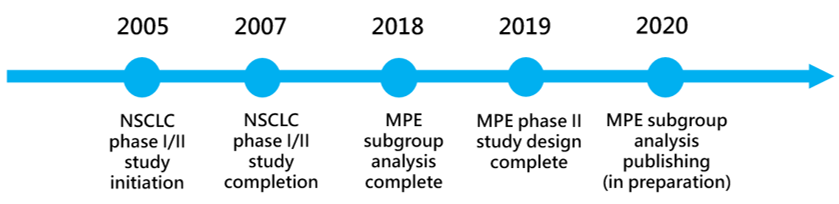
Pipeline Development Milestones
Gongwin Biopharm has planned a phase two, multi-center, open label clinical trial to evaluate the safety and efficacy of PTS products for the treatment of patients with malignant pleural effusion. This project is expected to be conducted in Australia.